Medical Device Co-Design Studio
The Translational Innovation Program of the CCTS, in partnership with Design, Biomedical Engineering, M4Lab and other partners, presents a graduate-level, project-based experiential course on medical device development. In addition to using the co-design process to ascertain the human potential of the invention, the early technology development course will nurture entrepreneurial capacities and mindsets in participating students and faculty. Students will learn how to translate technical, scientific or creative concepts to innovative business ideas & to create value for patients and people who are the ultimate beneficiaries.
Funded by NIH National Center for Advancing Translational Science (NCATS), the CCTS Optimizing Innovation, Commercialization and Entrepreneurship (O.I.C.E.) The program aims to support the mandate from the National Institutes of Health (NIH) and NCATS to move technology forward towards real-world impact through the entrepreneurship of research in an enabling environment as one pathway. CCTS O.I.C.E. Program works closely with Ohio State’s Corporate Engagement Office and Nationwide Children’s Hospital’s Office of Technology Commercialization along with a growing number of partners and co-supporters towards our shared mission to nurture academic entrepreneurship.
Motivated and creative graduate students from various disciplines with a passion for entrepreneurship will apply design principles and co-design approaches on 3 institution-owned (Ohio State or NCH) breakthrough technologies along with their respective inventors within teams.
This one semester, project-based experiential learning opportunity will offer 3 customized co-design workshops for each medical device to demonstrate how biomedical innovations evolve through successive iterations from concept to clinical adoption. Students will also learn how to clarify and prioritize clinical needs, value creativity in ideation, address FDA regulation and begin prototyping to understand how co-creation with end-users is critical for real-world impact and success.
Who is an academic entrepreneur? Faculty, staff or students turning observations in the laboratory, clinic, and community into interventions that improve the health of individuals and the public and seeking to:
- patent and/or license their work
- spin-out or spin-in ventures based on evidence
- collaborate with industry to realize impact
As a catalyst for inclusive entrepreneurship, we strive to synergize and support the collective diversity, equity and belonging efforts of The Ohio State University towards the elimination of racism in all forms. We will intentionally invite, showcase, nurture and increase participation in innovation, commercialization and entrepreneurship by all people and seek to address underrepresentation among women, people of color, people with disabilities and socially or economically disadvantaged persons.
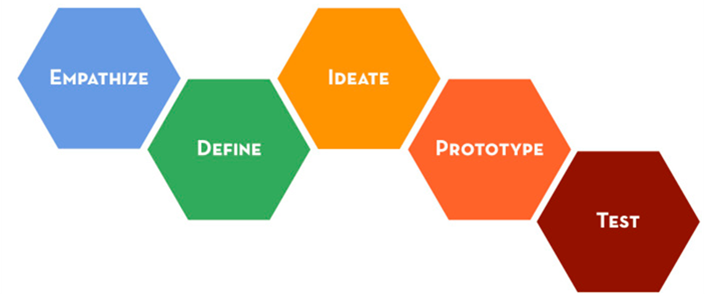
Stanford Design Thinking Process
What is Co-Design?
Co-design is an approach to design that actively involves all stakeholders in the design and development process to ensure the resulting product or device is simultaneously useful, usable, feasible, viable and desirable. In the Co-Design Studio we will explore the human/social potential for your invention in collaboration with you. We will use a human-centered approach to investigate what value your invention will bring to those who would be impacted by its introduction to the market. The question is not yet one of monetary value. We will first explore the use/experience value and the social value of the invention to make sure that we are making the right product. The next stage will be for you to establish the invention’s commercial value before working to make the product right.
The first question we will ask is this invention useful?
- Does it address people’s unmet needs? Which needs?
- Does it solve problems for people? How?
- Does it change people’s lives for the better? How?
- Are similar products already available? If so, how is this one different?
The second question: is this invention usable?
- Does it fit the user physically, cognitively and socially?
- Can the user readily learn to use it?
- Can the user easily use it?
- Is the user-device interface intuitive? Is it well-designed?
- Can the user set-up, clean, maintain and fix the device?
- Is it safe to use?
The third question: is this invention desirable?
- How do people feel when using it?
- Do people enjoy using it?
- Does the user want to use it?
- Does the user want to own it?
Once the human/social potential of your invention has been established, it is time to explore the invention’s commercial potential. The knowledge and insights gained in the Co-Design Studio will help you to prepare your case for describing the value proposition and the commercial viability of your invention.
Who should be involved in co-design?
Everyone who has the potential to impact the design as well as everyone who will be impacted by what is designed should ideally be involved. For medical devices, the people who have the potential to impact the design include all members of the project team together with other stakeholders who will be involved in designing, engineering, manufacturing, marketing, distributing, and selling the device. Those who will be impacted by the design include medical professionals, patients, caregivers, technologists, trainers, etc. Of course, it is not possible to engage all these potential co-designers, but the aim is to invite, include and involve a very diverse set of stakeholders customized to each device.
How can the Co-Design Approach Impact Your Invention?
- Reduce the risk of failure
- Better device quality in terms of usefulness, usability, desirability and feasibility
- Harness and integrate the contributions of multiple stakeholder perspectives
- Obtain relevant feedback from the co-designers before it is too late to use their input
- Participation can build ownership in the product/device
- Your device will be better able to contribute to just and equitable health futures.
Goals of the course are to:
- Accelerate the development of up to 3 early-stage medical devices with the help of a transdisciplinary team of graduate students and stakeholders (customized to each device) in the front end of the design process to ensure that the device is useful, usable, feasible and desirable.
- Provide 3 customized, co-design workshops to the inventors for fresh insights that can be used to attract seed funding, enhance market validation and fine-tune proof of concept in collaboration with stakeholders in the context of their needs and priorities.
- Co-create innovative entrepreneurship curriculum that leverages the diversity of disciplines, domains, thinking styles, and outcome orientation of various experts at The Ohio State University & Nationwide Children’s Hospital
- Understand the variations in complexity, purposes (therapeutic, diagnostic, monitoring, supportive, surgical, etc.) and timelines that affect the medical device development cycle of different types of medical devices and their path of technology commercialization.
- Demystify and nurture an entrepreneurial mindset and co-design skills among participating inventors and students for real-world applications in their respective careers
- Build students’ capability and confidence as inclusive problem solvers, empathic communicators, engaged collaborators and agile leaders
- Promote entrepreneurship as an exciting pathway and outcome of university research
- Basic understanding of types of regulatory oversight needed for devices intended for human use and design controls to prove the medical device's usability, safety and effectiveness.
Key Dates
Release Date: December 22, 2020
Application Due Date: January 12, 2021, midnight
Notice of Selection of Device: January 15, 2021
Inventor Pitch Presentations: January 25, 2021
Co-Design Workshop 1: February 15, 2021
Co-Design Workshop 2: March 8, 2021
Co-Design Workshop 3: March 29, 2021
Final Presentation Date: April 19, 2021
Please feel free to email questions to Dr. Tanya Mathew, CCTS or Dr. Liz Sanders, Design.
If you are an inventor or aspiring inventor of a medical device with IP, please apply here.
Request for Applications & Useful Resources
CCTS Medical Device Co-Design RFA for Inventors
CCTS Medical Device Co-Design Flyer for Graduate Students
The Fundamental Characteristics of a Translational Scientist
How to apply a design thinking, HCD, UX or any creative process from scratch | by Dan Nessler | Digital Experience Design | Medium
Convivial Toolbox: Generative Research for the Front End of Design by Dr. Liz Sanders
Briefly, well known designers talk about the importance of execution, human centered design thinking, and how the creative brief is iterative, ever dynamic.
U.S. FDA’s Device Advice: Comprehensive Regulatory Education Online Education Tool September 4, 2020
Current Trends And Approaches To Manage Your Medical Device Regulatory Content: Webinar October 7, 2020