Support for ClinicalTrials.gov Compliance
ClinicalTrials.gov is a database of privately and publicly funded clinical studies providing information about clinical trials to patients, family members, health care professionals, researchers and the larger public. Investigators are responsible for compliance with all regulations related to public disclosure of clinical trial information.
Failure to comply with this policy can lead to financial penalties, withholding of federal grant money or requirement to pay money back, and/or inability to publish results. It is Ohio State policy that clinical trials must be registered and summary results reported on ClinicalTrials.gov in accordance with federal regulations and NIH policy.
This site hosts information for Administrators and Investigators to help support compliance with ClinicalTrials.gov regulations.
The ClinicalTrials.gov Protocol Registration System is the portal used to manage clinical trial information.
Information for Administrators
This document provides a summary of responsibilities of an organization's ClinicalTrials.gov Protocol Registration and Results System (PRS) Administrator. New administrators may wish to use the Quick Start Guide to learn the basics of creating and maintaining records before reading this guide.
1. Create user/administrator accounts.
Create accounts for users and other administrators in your organization as needed, using the New User Account option from the Accounts menu on the Home page. Users create and modify their own records, but normally cannot access other users' records. Administrators have full access to all records within the organization. Study investigators can be given either user or administrator accounts at the discretion of each organization.
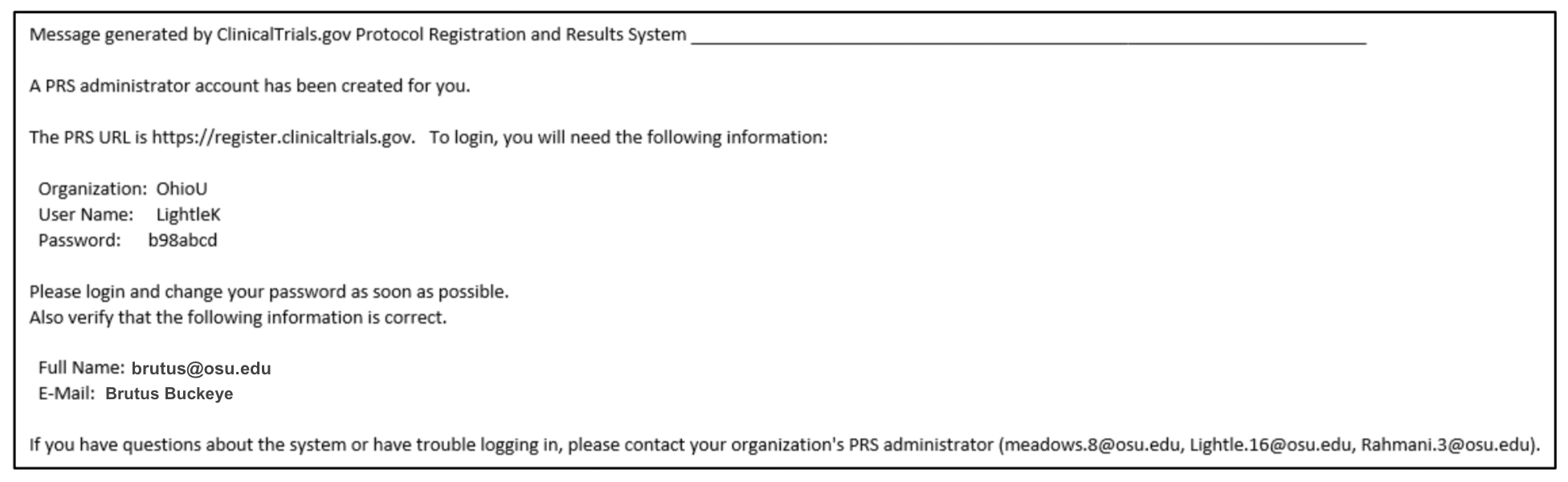
The PRS automatically sends email with the PRS web address and login information to the user/administrator upon account creation.
2. Review, Approve and Release completed records.
Whenever a user marks a new or modified record as "Entry Completed," the administrator is responsible for reviewing the record. Open the record and review all content, making corrections as needed. Follow instructions in the "Next Step" box on the "Record Summary" page to "Approve and Release the record for PRS Review."
IMPORTANT: Pay special attention to recruiting status and contact information when reviewing records, as the accuracy and timeliness of this information is extremely important to patients and health care providers.
All records must be free of ERROR messages in order to be released.
EXCEPTION: If the Investigator is designated as Responsible Party for a study, that individual has the authority (and responsibility) to Approve and Release the record even if not an administrator. In that case administrators can "Approve" the record but cannot "Release" it. More information on Responsible Party is accessible from the "Edit Sponsor/Collaborators page."
3. Ensure that records are kept up to date.
The administrator has overall responsibility to ensure that the organization's records are verified, updated and re-released as needed (at least yearly while a study is active).
Email messages are automatically sent by the PRS to administrators for significant user actions, such as completing data entry for a record or modifying a previously released record.
4. Check for problems.
Use the "Problems" column on the Home Page to identify important issues, such as records that were updated but never released or records that are missing information required by the U.S. FDA Amendments Act (FDAAA 801).
Work with the owner of each problem record as needed to address the issues, following instructions in the "Next Step" box on the "Record Summary" page to complete the process of updating the record.
5. Maintain user accounts.
Reset passwords, update email addresses or make other user account modifications via the "Accounts Menu" on the "Home Page."
6. Disable user accounts.
Disable a user's account using the "Enable/Disable User Account" option under the "Accounts" menu on the Home Page. This is often necessary when a user leaves the organization or no longer requires access to the PRS.
7. (Optional): Maintain Access Lists for records.
A record owner or PRS administrator can optionally grant access to a record, to one or more additional users within the same organization, using the Edit Access List link on the Record Summary page. Users granted access to a record in this manner have the same privileges as the record owner, except for modification of the Access List.
This document provides a summary of responsibilities of an organization's ClinicalTrials.gov Protocol Registration and Results System (PRS) Administrator.
Administrators have three primary responsibilities:
- Maintain their organization's PRS account, including monitoring for problems with records
- Approve and release study records and updates when the Sponsor is the Responsible Party
- Serve as the primary point of contact with the ClinicalTrials.gov team
Each college will have at least one designated CT.gov PRS administrator that will be the point person for the college. Additional administrators can be added if the level of work warrants additional administrators.
Administrator accounts can be created by the college admin or requested from Sandra Meadows.
Logging into the System
Protocol Registration and Results System Login is not found on ClinicalTrials.gov, the login is on the registration page.
Responsibilities of the Administrator
Create user/administrator accounts.
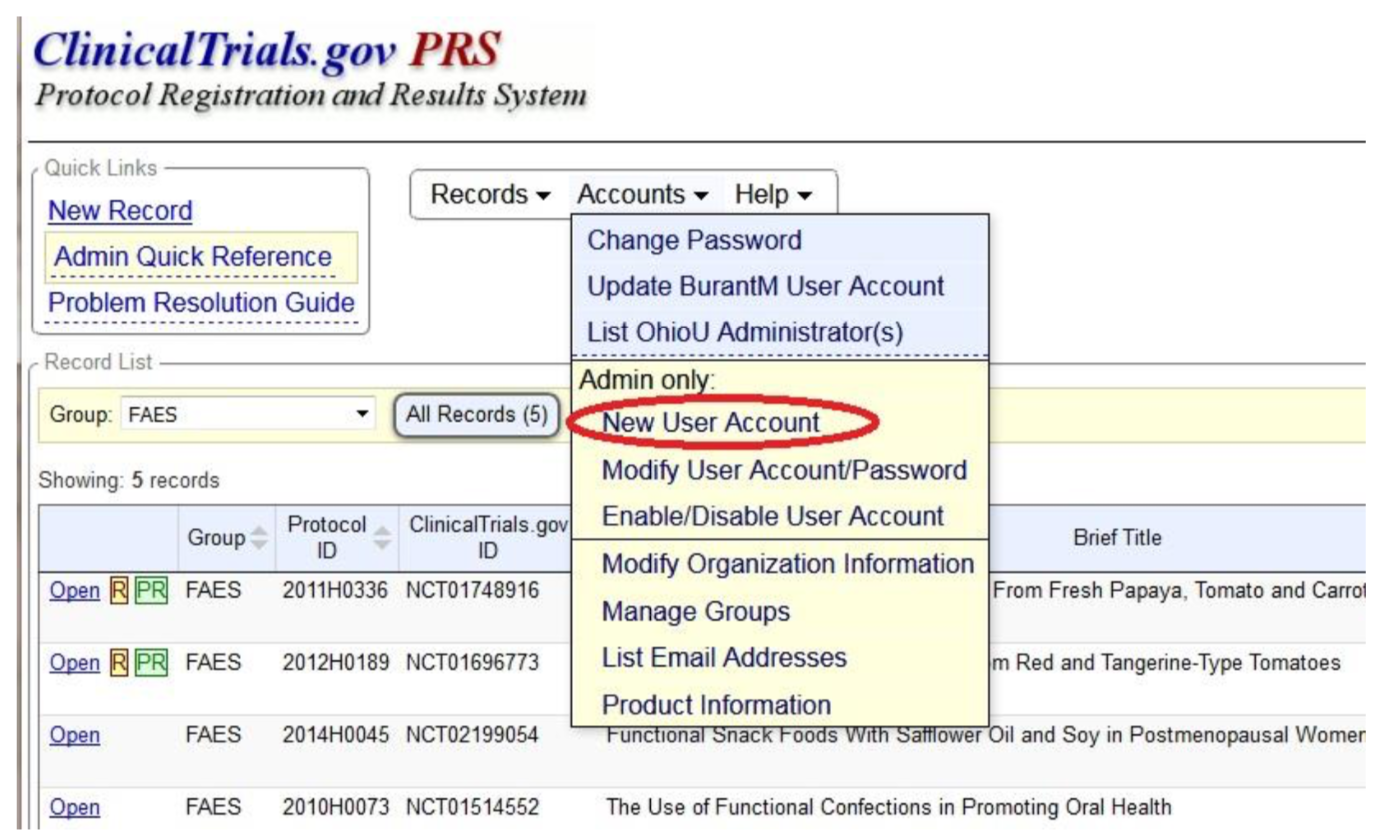
PRS Administrators create accounts for users in their college as needed, using the New User Account option from the Accounts menu on the top of the Clinicaltrials.gov PRS Home Page.
- The PRS automatically sends email with the PRS web address and login information to the user/administrator upon account creation.
- Administrators have full access to all records within the organization.
- Users create and modify their own records but normally cannot access other users' records.
- Study investigators are provided user accounts, not administrator accounts.
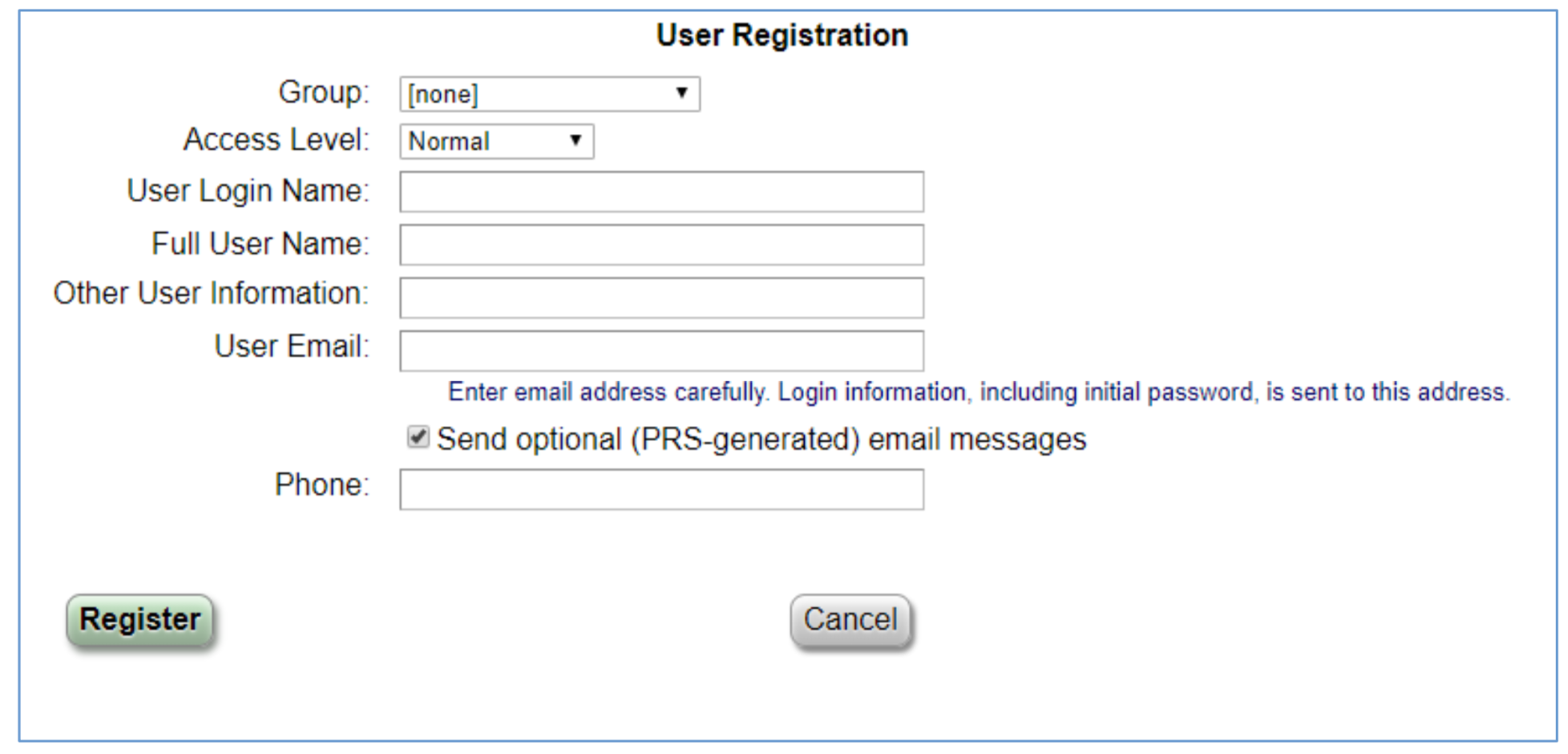
Best Practices for Filling in the Fields:
Group: Choose college from dropdown
Access Level: Normal
User Login Name: Ohio State name.#
Full User Name: First Last
Other User Information: Can put anything in this field, department is an option
User Email: Ohio State email
Send Optional (PRS-generated) email message: leave box checked
Phone: Ohio State Phone
Copy of email that new user receives from the PRS System:
Resources
Review, Approve and Release completed records
Whenever a user marks a new or modified record as Entry Completed, the PRS administrator is responsible for reviewing the record.
- Open the record and review all content, making corrections as needed.
- Follow instructions in the "Next Step" box on the "Record Summary" page to "Approve and Release" the record for PRS Review.
- Pay special attention to recruiting status and contact information when reviewing records, as the accuracy and timeliness of this information is extremely important to patients and health care providers.
- All records must be free of ERROR messages in order to be released.
- EXCEPTION: If the Investigator is designated as Responsible Party for a study, that individual has the authority (and responsibility) to "Approve and Release" the record even if not an administrator. In that case administrators can approve the record but cannot release it. More information on Responsible Party is accessible from the "Edit Sponsor/Collaborators" page.
Directions
Updating and Maintaining Records
Ensure that records are kept up to date
The administrator has overall responsibility to ensure that the organization's records are verified, updated and re-released as needed (at least yearly while a study is active).
- Email messages are automatically sent by the PRS to administrators for significant user actions, such as completing data entry for a record or modifying a previously released record.
- View the record specified in emails messages and check for problems.
- Use the Problems column on the Home page to identify important issues, such as records that were updated but never Released or records that are missing information required by the U.S. FDA Amendments Act (FDAAA 801).
- Work with the owner of each problem record as needed to address the issues, following instructions in the "Next Step" box on the Record Summary page to complete the process of updating the record.
- There are three basic reports available in PRS – "Home Page Report", "Public Site Report", and" Planning Report." Detailed information on how to access and best uses for all three can be found in the Introduction to CT.gov Reports document. Scheduling a time each month to review reports and follow up with record owners is a best practice.
Directions
Problems with Records and How to Resolve
Maintain user accounts
- Reset passwords via the "Accounts" menu on the Home page.
- Update email addresses or make other user account modifications via the "Accounts" menu on the Home page
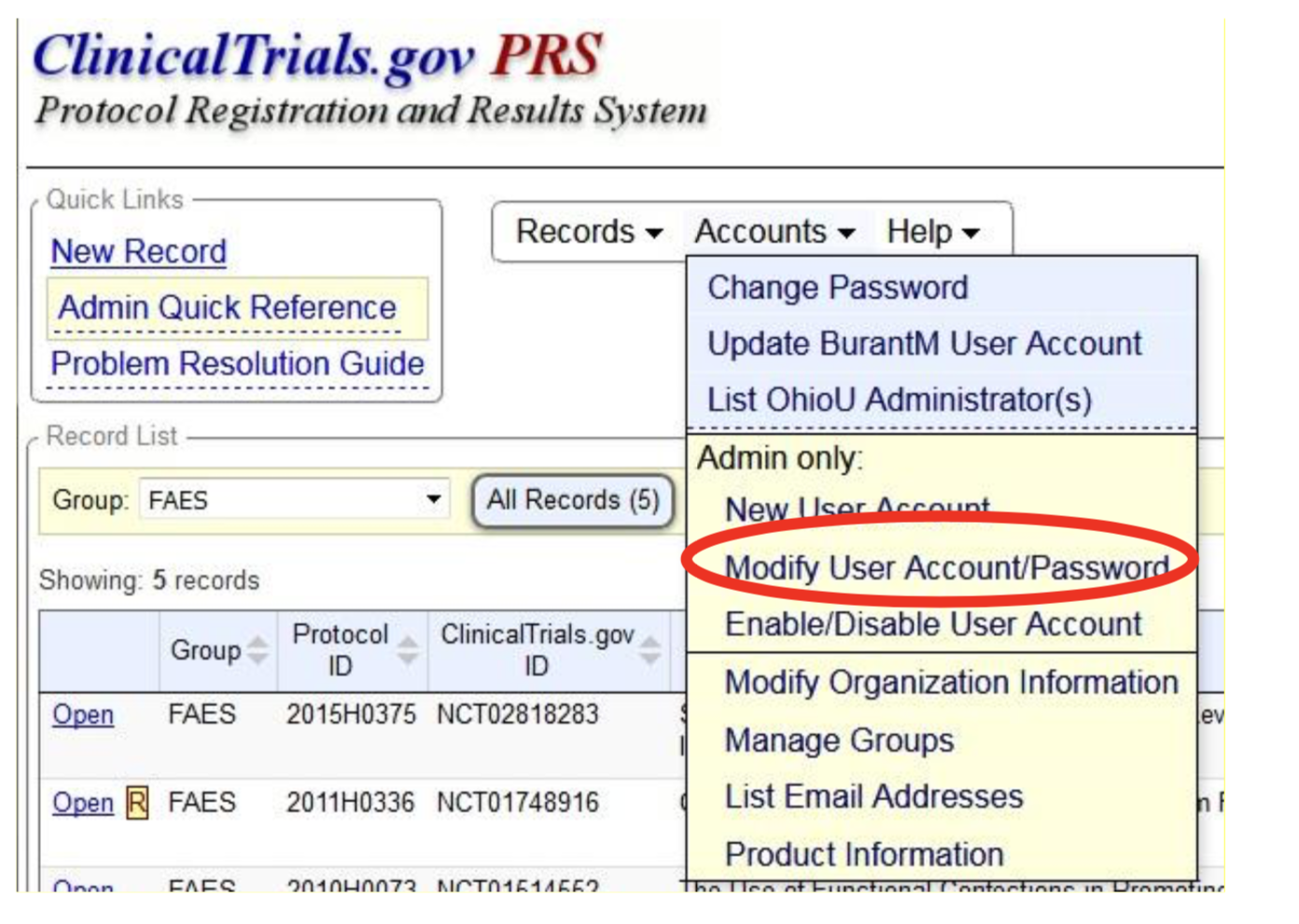
Update/Modify User Account Information
1. Click on "Modify User Account/Password" from the "Admin Only" section of the "Accounts"
2. Click on "Modify" next to the username for the account to be updated.
3. Enter changes on the "User Information" page.
4. Click "Save."
Change User Password
1. Click on "Modify User Account/Password" from the "Admin Only" section of the "Accounts" menu
2. Click on "Reset Password" next to the username for which the password is to be reset.
3. Enter and confirm a new password.
4. Click on "Reset Password." The new password will be emailed automatically to the user.
Directions
Update/Modify User Account Information
Disable user accounts
Disable a user's account using the "Enable/Disable User Account" option under the "Accounts" menu on the Home Page. This is often necessary when a user leaves the organization or no longer requires access to the PRS. Also, when users have multiple accounts.
Directions to disable/enable User Accounts
Maintain Access Lists for records
A record owner or PRS administrator can optionally grant access to a record, to one or more additional users within the same organization, using the "Edit Access List" link on the "Record Summary" page. Users granted access to a record in this manner have the same privileges as the record owner, except for modification of the "Access List."
- Overview
ClinicalTrials.gov, a service of the National Institutes of Health (NIH), was developed by the United States National Library of Medicine in collaboration with the Food and Drug Administration (FDA). The service was developed to “link patients to medical research” and to provide information to the general public about federally and privately supported clinical research for a range of diseases and conditions. Registration of clinical trials and the subsequent results reporting in the Protocol Registration and Results System (PRS) of ClinicalTrials.gov is required by federal guidelines and NIH policy. Although not covered in this policy, there may be other requirements by the International Committee of Medical Journal Editors (ICMJE) or the Center for Medicare and Medicaid Services (CMS). The investigator is responsible for ensuring compliance with such requirements. It is Ohio State policy that clinical trials must be registered and summary results reported on ClinicalTrials.gov in accordance with federal regulations and NIH policy.
- Definitions
Applicable clinical trial (42 CFR 11) or ACT: A controlled clinical investigation, other than a phase I clinical investigation, of a drug or biological product subject to FDA regulation; or a prospective clinical study of health outcomes comparing an intervention with a device product subject to FDA regulation, other than a small clinical feasibility study.
Clinical trial (NIH): A research study in which one or more human participants are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Note: This definition encompasses phase I trials of FDA-regulated drug and biological products, small feasibility studies of FDA-regulated device products, and studies of any intervention not regulated by the FDA (e.g., behavioral intervention).
Note: This definition of “clinical trial” is broader than the term “applicable clinical trial” (ACT) as defined in the regulation (i.e., 42 CFR 11).
Principal Investigator (42 CFR 11): The individual who is responsible for the overall scientific and technical direction of the study.
Primary completion date: The date that the final participant in a clinical study was examined or received an intervention for the purposes of final collection of data for the primary outcome, whether the clinical trial concluded according to the pre-specified protocol or was terminated.
Responsible party: The sponsor of the clinical trial, or the principal investigator of such clinical trial if so designated by a sponsor, grantee, contractor, or awardee, so long as the principal investigator is responsible for conducting the trial, has access to and control over the data from the clinical trial, has the right to publish the results of the trial, and has the ability to meet all of the requirements for the submission of clinical trial information. Note: For research initiated by an Ohio State investigator, the principal investigator is the responsible party unless the study sponsor retains clinical trial registration responsibilities.
Sponsor (FDA 21 CFR 50.3): A person who initiates a clinical investigation, but who does not actually conduct the investigation, i.e., the test article is administered or dispensed to or used involving, a subject under the immediate direction of another individual. A person other than an individual (e.g., corporation or agency) that uses one or more of its own employees to conduct a clinical investigation it has initiated is considered to be a sponsor (not a sponsor-investigator), and the employees are considered to be investigators.
Sponsor-Investigator (FDA 21 CFR 50.3): An individual who both initiates and actually conducts, alone or with others, a clinical investigation (i.e., under whose immediate direction the test article is administered or dispensed to, or used involving, a subject). The term does not include any person other than an individual (e.g., corporation or agency).
- Clinical Trials Requirements
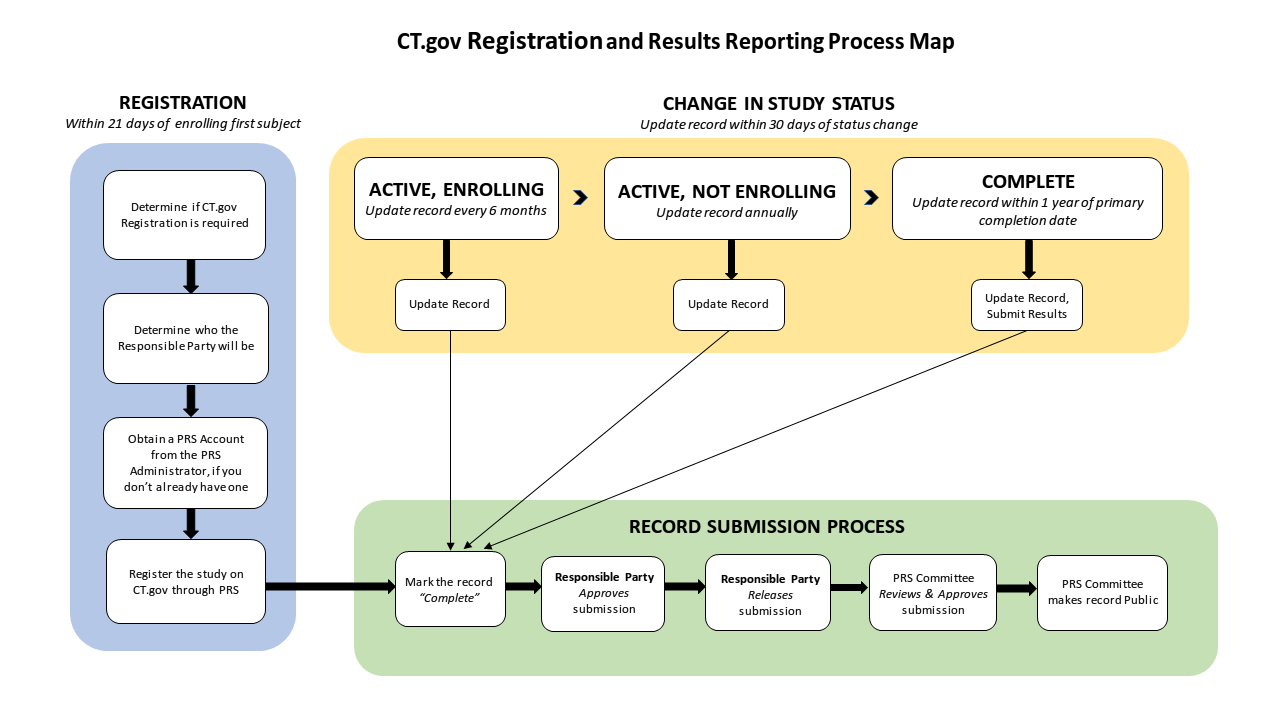
- Registration
- Responsible parties must register clinical trials as outlined below:
- 42 CFR 11 – within 21 days of enrolling first participant
- NIH – within 21 days of enrolling first participant
- For further registration guidance, see ClinicalTrials.gov.
- FDA regulations and NIH policy require informed consent processes and documents for ACTs involving drugs, biologics, and devices and clinical trials respectively, to include a specific statement that clinical trial information will be entered into ClinicalTrials.gov. See HRPP policy, Informed Consent Process and Elements of Informed Consent, for more information.
- Responsible parties must register clinical trials as outlined below:
- Updates
All active studies must be updated annually; every six months if the study is recruiting subjects. Recruitment and enrollment status changes and trial closure must be entered within 30 days.
- Results Reporting
- Final study results of 42 CRF 11 ACTs and/or NIH-supported clinical trials are required to be reported no later than one year after the primary completion date.
- Responsible parties are also required to post the study protocol and statistical analysis plan (if not included in the protocol), as well as a description of the quality assurance procedures used throughout the trial at the time of results reporting.
- For further results reporting guidance, see ClinicalTrials.gov.
- Registration
- Departmental Support
All colleges conducting reportable clinical trials must appoint a designated ClinicalTrials.gov administrator to support registration activities of Ohio State-initiated clinical research and oversee timely results reporting by the responsible party. Refer to college or department standard operating procedures for additional guidance on ClinicalTrials.gov registration and reporting requirements.
If the PI is the responsible party and leaves the institution after study completion, the study data should remain at Ohio State to ensure that results reporting is completed. See Research Data Policy for further information. Alternatively, the study record will be transferred to the PI’s new institution for continued oversight.
- Institutional Reporting
Clinical trial registration and results reporting by colleges/institutes/centers will be made available to university officials. Quarterly reports will be provided to the Office of Research with Institutional Official (IO) notification of on-going noncompliance, as applicable.
- Noncompliance with Registration and Results Reporting Requirements
Responsible parties who fail to comply with ClinicalTrials.gov registration and/or results reporting within required time frames of study initiation/completion will be reported to the applicable College Research Officer or designee. Continued noncompliance with ClinicalTrials.gov requirements will result in IO notification and possible loss of PI status, and may result in civil monetary penalties, and for federally-funded trials, withholding or recovery of grant funds.
- Applicable Regulations/Guidance
42 CFR Part 11, NIH “Clinical Trials Registration and Results Information Submission” (9/21/16), 21 CFR 50, Informed Consent Elements (1/4/11), FDAAA U.S. Public Law 110-85 (9/27/07), ClinicalTrials.gov (12/18/17).
Information for Investigators
There are three basic reports available in PRS:
- Home Page Report: This report is useful for managing the registration and problem resolution processes
- Public Site Report: This report lists those studies available to the public along with the basic status of each
- Planning Report: This report helps administrators manage upcoming required actions, such as updates and results reporting deadlines
The Home Page Report is automatically loaded to the home view after the user/administrator logs in. The other reports are accessible in the drop down "Records" at the top of the page:
Home Page Report
The report that automatically loads after logging into PRS has no official name; in this document it is called the Home Page Report. This report shows registered studies and what stage they are in (in progress through terminated/completed), as well as problems PRS has noted for each. Users can show and hide columns, filter the data, and export to Excel.
Tips
- Use the "Group" drop-down to select the unit/college of interest. The buttons to the right of the "Group" dropdown clear filters, filter for problem records, or allow the administrator to build a custom filter.
- An administrator can review all entries and adjust the view by using the show/hide columns button or sorting by a column. The show/hide columns button allows you to add a wide variety of non-standards columns.
- The information can be downloaded to a spreadsheet. Only the data selected at that point will be downloaded (i.e. filter and column choices are maintained). The responsible party information includes the name and email address in the same cell.
Suggested Filters
- Look for records with a problem
- Click the "Problem Records" button to see only those records with any problem.
- Use the "Custom Filter button" to specify the problem type of interest (not recently updated, for instance) or to remove records that PRS in reviewing (select all "Record Statuses except "PRS review".")
- Records with problems that are also public may be of greater compliance interest.
- Records that are in progress and have no had recent progress may deserve follow-up.
- Find all NIH-funded research by using Show/Hide Columbus button to include "Secondary IDs". Federal grant identifiers should be included in this field.
Data Elements: Home Page Report
|
Data Element |
Comment |
|
Group |
Ohio State-defined |
|
Protocol ID |
Any unique number assigned to protocol by sponsor. (Ohio State protocol ID) |
|
clinicaltrials.gov ID |
NCT# |
|
Brief Title |
Title intended for the public |
|
Record Status |
Approved/Public/In Progress/Released/No Longer Public |
|
Last Update |
Date is manually changed by those who can access the record |
|
Record Owner |
Creator |
|
Responsible Party |
Name, email address |
|
Problems |
|
|
Secondary IDs |
Federal Grant #s required; other IDs may be included. 30 characters |
|
Overall Status |
Active, not recruiting/Completed/Enrolling by invitation/Not yet recruiting/Recruiting/Terminated |
|
Verification Date |
|
|
Primary Completion Date |
The date that the final participant was examined or received an intervention for the purposes of final collection of data for the primary outcome |
|
Results status |
|
|
Delayed Results Status |
|
|
Last Updated by |
Username for PRS staff |
|
Central Contacts |
Shows all contacts |
|
Study Officials |
Shows all contacts |
Public Site Report
This report essentially filters studies by whether they are public on ClinicalTrials.gov or not and provides information related to public release of information on the website. Only studies that have passed PRS review will be made public.
Use the "Records" dropdown at the top of the page to select "Public Site Report" to access this report.
Tips
- Use the drop-down boxes to select the group of interest and public site status (Public/Not Public/All). Use the Show/Hide Columbus to customize the view. This screenshot shows all available columns.
- The information can be downloaded to a spreadsheet. (The button is at the bottom of the page) Only the data selected at that point will be downloaded (i.e. filter and column choices are maintained.)
Suggested Uses
- This report shows the administrator what the larger public is able to see and therefore may be important for managing reputation. Use this report to track the number of studies by status, including enrollment status. Track the number of studies that have reported results.
Data Elements - Public Site Report
Planning Report
|
Data Element |
Comment |
|
Protocol ID |
Any unique number assigned to protocol by sponsor |
|
Brief Title |
Title intended for the public |
|
Public Site Status |
Protocol registered, No longer public, Results posted |
|
Overall Status |
Active, not recruiting/Completed/Enrolling by invitation/Not yet recruiting/Recruiting/Terminated |
|
Study Type |
Interventional, observational, or expanded access |
|
Phase |
|
|
Initial Release |
Date record was first released to public site |
|
Initial Results Release |
Date results were first released to public site |
|
Last Public Release |
Time stamp (mm/dd/yyy) for last update to record |
|
Last Modifier |
User name of last person to modify record |
|
Verification Date |
The date on which the responsible party last verified the clinical study information in the entire ClinicalTrials.gov record for the clinical study, even if no additional or updated information is being submitted. Month and year only. Must be manually set in Study Status section. |
|
Primary Completion Date |
The date that the final participant was examined or received an intervention for the purposes of final collection of data for the primary outcome. (Actual or Anticipated is noted parenthetically) |
|
Study Completion Date |
The date the final participant was examined or received an intervention for purposes of final collection of data for the primary and secondary outcome measures and adverse events (Actual or Anticipated is noted parenthetically) |
Planning Report
Use the Records dropdown to select "Planning Report." This report is used to see when record updates and results reporting are expected for each record. The planning report is not useful for tracking records that need attention - it does not contain the "problems" column of the public site report.
Tips
- In the planning report, you can choose the group and use the buttons to toggle among all records, those with an expected action, or a custom filter.
- Show/hide columns is also available to customize the view. The show/hide columns button allows you to add a wide variety of non-standard columns.
- The planning report can be downloaded to a spreadsheet. Only the data selected at that point will be downloaded (ie: filter and column choices are maintained). It loads the record owner's name and email address into the same cell, however.
- Note that the FDAAA column showing "Applicable Clinical Trials and Presumed Applicable Clinical Trials" is informational only - these tags are generated using machine logic, but the Responsible Party makes the final determination. Click on the hyperlink to see the record details that cause the system to identify the trial as an applicable clinical trial (ACT), a probable ACT (pACT), or neither (blank).
- The custom filter has a wide variety of options for tracking upcoming required actions.
PHOTO
Suggested Filters
- Add the "NIH Grants" column to identify those records that either list an NIH Grant number or include an NIH institute/center as a collaborator.
- Use the "Action Expected" button to filter out records with no action required at the time.
- Pay close attention to the "Update Expected", "Corrections Expected", "Results Expected", and "All Results Expected" columns.
Data Elements/ Planning Report
|
Data Element |
Comment |
|
Protocol ID |
Any unique number assigned to protocol by sponsor |
|
Brief Title |
Title intended for the public |
|
Public Site Status |
Protocol registered, No longer public, Results posted |
|
Overall Status |
Active, not recruiting/Completed/Enrolling by invitation/Not yet recruiting/Recruiting/Terminated |
|
Study Type |
Interventional, observational, or expanded access |
|
Phase |
|
|
Initial Release |
Date record was first released to public site |
|
Initial Results Release |
Date results were first released to public site |
|
Last Public Release |
Time stamp (mm/dd/yyy) for last update to record |
|
Last Modifier |
User name of last person to modify record |
|
Verification Date |
The date on which the responsible party last verified the clinical study information in the entire ClinicalTrials.gov record for the clinical study, even if no additional or updated information is being submitted. Month and year only. Must be manually set in Study Status section. |
|
Primary Completion Date |
The date that the final participant was examined or received an intervention for the purposes of final collection of data for the primary outcome. (Actual or Anticipated is noted parenthetically) |
|
Study Completion Date |
The date the final participant was examined or received an intervention for purposes of final collection of data for the primary and secondary outcome measures and adverse events (Actual or Anticipated is noted parenthetically) |
Planning Report
Use the Records dropdown to select "Planning Report". This report is used to see when record updates and results reporting are expected for each records. The planning report is not useful for tracking records that need attention. It does not contain the "problems" column of the public site repot.
Tips
- In the planning report you can choose the group and use the buttons to toggle among all records, those with an expected action, or a custom filter.
- Show/hide columns is also available to customize the view. The show/hide columns button allows you to add a wide variety of non-standard columns.
- The planning report can be downloaded to a spreadsheet. Only the data selected at that point will be downloaded (ie: filter and column choices are maintained). It loads the records owner's name and email address into the same cell, however.
- Note that the FDAAA column showing Applicable Clinical Trials and Presumes Applicable Clinical Trials is informational only - these tags are generated using machine logic, but the Responsible Party makes the final determination. Click on the hyperlink to see the record details that causes the system to identify as an applicable clinical trial (ACT), a probable ACT (pACT), or neither (blank).
- The custom filter has a wide variety of options for tracking upcoming required actions:
Suggested Filters
- Add the "NIH Grants" column to identify those records that either list an NIH grant number or include an NIH institutes/center as a collaborator.
- Use the "Action Expected" button to filter out records with no action required at the time.
- Pay close attention to the "Update Expected", "Corrections Expected", "Results Expected", and "All Results Expected" columns.
Data Elements - Planning Report
|
Data Element |
Comment |
|
Protocol ID |
Any unique number assigned to protocol by sponsor |
|
Brief Title |
Title intended for the public |
|
Public Site Status |
Protocol registered, No longer public, Results posted |
|
Overall Status |
Active, not recruiting/Completed/Enrolling by invitation/Not yet recruiting/Recruiting/Terminated |
|
Study Type |
Interventional, observational, or expanded access |
|
Phase |
|
|
Initial Release |
Date record was first released to public site |
|
Initial Results Release |
Date results were first released to public site |
|
Last Public Release |
Time stamp (mm/dd/yyy) for last update to record |
|
Last Modifier |
User name of last person to modify record |
|
Verification Date |
The date on which the responsible party last verified the clinical study information in the entire ClinicalTrials.gov record for the clinical study, even if no additional or updated information is being submitted. Month and year only. Must be manually set in Study Status section. |
|
Primary Completion Date |
The date that the final participant was examined or received an intervention for the purposes of final collection of data for the primary outcome. (Actual or Anticipated is noted parenthetically) |
|
Study Completion Date |
The date the final participant was examined or received an intervention for purposes of final collection of data for the primary and secondary outcome measures and adverse events (Actual or Anticipated is noted parenthetically) |
Planning Report
Use the Records dropdown to select "Planning Report". This report is used to see when record updates and results reporting are expected for each record. The planning report is not useful for tracking records that need attention - it does not contain the "problems" column of the public site report.
Tips
- In the planning report, you can choose the group and use the buttons to toggle among all records, those with an expected action, or a custom filter.
- Show/hide columns is also available to customize the view. The show/hide columns button allows you to add a wide variety of non-standard columns.
- The planning report can be downloaded to a spreadsheet. Only the data selected at that point will be downloaded (ie: filter and column choices are maintained). It loads the record owner's name and email address into the same cell, however.
- Note that the FDAAA column showing Applicable Clinical Trials and Presumed Applicable Clinical Trials is informational only - these tags are generated using the machine logic, but the Responsible Party makes the final determination. Click on the hyperlink to see the record details that cause the system to identify the trial as an applicable clinical trial (ACT), a probabl ACT (pACT), or neither (blank).
- The custom filter has a wide variety of options for tracking upcoming required actions:
Suggested Filters
- Add the "NIH Grants" column to identify those records that either list an NIH grant number or include an NIH institute/center as a collaborator.
- Use the "Action Expected" button to filter out records with no action required at the time.
- Pay close attention to the "Update Expected", "Corrections Expected", "Results Expected", and "All Results Expected" columns.
Data Elements - Planning Report
- Pay close attention to the "Update Expected", "Corrections Expected", "Results Expected", and "All Results Expected" columns.
- Overview
ClinicalTrials.gov, a service of the National Institutes of Health (NIH), was developed by the United States National Library of Medicine in collaboration with the Food and Drug Administration (FDA). The service was developed to “link patients to medical research” and to provide information to the general public about federally and privately supported clinical research for a range of diseases and conditions. Registration of clinical trials and the subsequent results reporting in the Protocol Registration and Results System (PRS) of ClinicalTrials.gov is required by federal guidelines and NIH policy. Although not covered in this policy, there may be other requirements by the International Committee of Medical Journal Editors (ICMJE) or the Center for Medicare and Medicaid Services (CMS). The investigator is responsible for ensuring compliance with such requirements. It is Ohio State policy that clinical trials must be registered and summary results reported on ClinicalTrials.gov in accordance with federal regulations and NIH policy.
- Definitions
Applicable clinical trial (42 CFR 11) or ACT: A controlled clinical investigation, other than a phase I clinical investigation, of a drug or biological product subject to FDA regulation; or a prospective clinical study of health outcomes comparing an intervention with a device product subject to FDA regulation, other than a small clinical feasibility study.
Clinical trial (NIH): A research study in which one or more human participants are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Note: This definition encompasses phase I trials of FDA-regulated drug and biological products, small feasibility studies of FDA-regulated device products, and studies of any intervention not regulated by the FDA (e.g., behavioral intervention).
Note: This definition of “clinical trial” is broader than the term “applicable clinical trial” (ACT) as defined in the regulation (i.e., 42 CFR 11).
Principal Investigator (42 CFR 11): The individual who is responsible for the overall scientific and technical direction of the study.
Primary completion date: The date that the final participant in a clinical study was examined or received an intervention for the purposes of final collection of data for the primary outcome, whether the clinical trial concluded according to the pre-specified protocol or was terminated.
Responsible party: The sponsor of the clinical trial, or the principal investigator of such clinical trial if so designated by a sponsor, grantee, contractor, or awardee, so long as the principal investigator is responsible for conducting the trial, has access to and control over the data from the clinical trial, has the right to publish the results of the trial, and has the ability to meet all of the requirements for the submission of clinical trial information. Note: For research initiated by an Ohio State investigator, the principal investigator is the responsible party unless the study sponsor retains clinical trial registration responsibilities.
Sponsor (FDA 21 CFR 50.3): A person who initiates a clinical investigation, but who does not actually conduct the investigation, i.e., the test article is administered or dispensed to or used involving, a subject under the immediate direction of another individual. A person other than an individual (e.g., corporation or agency) that uses one or more of its own employees to conduct a clinical investigation it has initiated is considered to be a sponsor (not a sponsor-investigator), and the employees are considered to be investigators.
Sponsor-Investigator (FDA 21 CFR 50.3): An individual who both initiates and actually conducts, alone or with others, a clinical investigation (i.e., under whose immediate direction the test article is administered or dispensed to, or used involving, a subject). The term does not include any person other than an individual (e.g., corporation or agency).
- Clinical Trials Requirements
- Registration
- Responsible parties must register clinical trials as outlined below:
- 42 CFR 11 – within 21 days of enrolling first participant
- NIH – within 21 days of enrolling first participant
- For further registration guidance, see ClinicalTrials.gov.
- FDA regulations and NIH policy require informed consent processes and documents for ACTs involving drugs, biologics, and devices and clinical trials respectively, to include a specific statement that clinical trial information will be entered into ClinicalTrials.gov. See HRPP policy, Informed Consent Process and Elements of Informed Consent, for more information.
- Responsible parties must register clinical trials as outlined below:
- Updates
All active studies must be updated annually; every six months if the study is recruiting subjects. Recruitment and enrollment status changes and trial closure must be entered within 30 days.
- Results Reporting
- Final study results of 42 CRF 11 ACTs and/or NIH-supported clinical trials are required to be reported no later than one year after the primary completion date.
- Responsible parties are also required to post the study protocol and statistical analysis plan (if not included in the protocol), as well as a description of the quality assurance procedures used throughout the trial at the time of results reporting.
- For further results reporting guidance, see ClinicalTrials.gov.
- Registration
- Departmental Support
All colleges conducting reportable clinical trials must appoint a designated ClinicalTrials.gov administrator to support registration activities of Ohio State-initiated clinical research and oversee timely results reporting by the responsible party. Refer to college or department standard operating procedures for additional guidance on ClinicalTrials.gov registration and reporting requirements.
If the PI is the responsible party and leaves the institution after study completion, the study data should remain at Ohio State to ensure that results reporting is completed. See Research Data Policy for further information. Alternatively, the study record will be transferred to the PI’s new institution for continued oversight.
- Institutional Reporting
Clinical trial registration and results reporting by colleges/institutes/centers will be made available to university officials. Quarterly reports will be provided to the Office of Research with Institutional Official (IO) notification of on-going noncompliance, as applicable.
- Noncompliance with Registration and Results Reporting Requirements
Responsible parties who fail to comply with ClinicalTrials.gov registration and/or results reporting within required time frames of study initiation/completion will be reported to the applicable College Research Officer or designee. Continued noncompliance with ClinicalTrials.gov requirements will result in IO notification and possible loss of PI status, and may result in civil monetary penalties, and for federally-funded trials, withholding or recovery of grant funds.
- Applicable Regulations/Guidance
42 CFR Part 11, NIH “Clinical Trials Registration and Results Information Submission” (9/21/16), 21 CFR 50, Informed Consent Elements (1/4/11), FDAAA U.S. Public Law 110-85 (9/27/07), ClinicalTrials.gov (12/18/17).

- Objective
To ensure that the Principal Investigator (PI) and all research team members assisting in the conduct of clinical research are informed about their obligations and responsibilities as they pertain to Good Clinical Practices (GCP), the investigational plan, applicable regulations, guidances, and institutional policies. This Standard Operating Procedure (SOP) applies to the written procedures followed by all members of a clinical research team involved in the conduct of human subjects’ research at The Ohio State University Wexner Medical Center (OSUWMC), hereafter called the investigational site. These detailed instructions promote compliance in conducting clinical research.
SOP-20 describes the process for the registration and results reporting of clinical trials to ClinicalTrials.gov. Attachment templates include:
A: Creating a New Study Record for ClinicalTrials.gov
B: Maintaining Study Records on ClinicalTrials.gov - Responsibility
The College of Medicine Clinical Trials Management Organization (COM-CTMO) develops, implements, and maintains SOPs. The need to write a new or revise an existing SOP is based upon changes to federal regulations, guidelines, institutional policies, or procedures. These documents will be provided to departments and research teams conducting human subjects’ research. Departments or research teams may develop additional research SOPs or a Research Procedure Addendum (RPA) to expand on an existing SOP, however this need should be limited.
The PI is ultimately accountable for all clinical research activities and is responsible for the appropriate delegation of tasks to individuals with adequate training and education to perform such tasks. It is the responsibility of all members of the clinical research team involved in supervising, managing, or conducting study-related activities to follow the SOPs. The clinical research team may include but is not limited to the following members:
Research Team Members- Principal Investigator (PI)
- Clinical Research Coordinator (CRC)
- Sub-Investigator (Sub-I)
- Clinical Research Assistant (CRA)
- Clinical Research Manager (CRM)
- Other Research Staff as appropriate
- Clinical Research Specialist (CRS)
- Administrative and Support Staff
- Definitions (as they pertain to registering a trial on ClinicalTrials.gov)
- Applicable Clinical Trial (ACT): Registration is required for trials that meet the FDAAA 801 definition of an "Applicable Clinical Trial" and were either initiated after September 27, 2007, or initiated on or before that date and were still ongoing as of December 26, 2007. Trials that were ongoing as of September 27, 2007 and reached their completion date (see Primary Completion Date data element on ClinicalTrials.gov) before December 26, 2007 are excluded. "Applicable Clinical Trials" include the following:
- Trials of drugs and biologics: Controlled clinical investigations, other than phase 1 clinical trials that do not fall under FDAMA, of drugs or biological products subject to Food and Drug Administration (FDA) regulation.
- Trials of devices: 1) Controlled trials with health outcomes of devices subject to FDA regulation, other than small feasibility studies where the primary outcome measure relates to feasibility and not to health outcomes, and 2) Pediatric Postmarket Surveillance required by FDA.
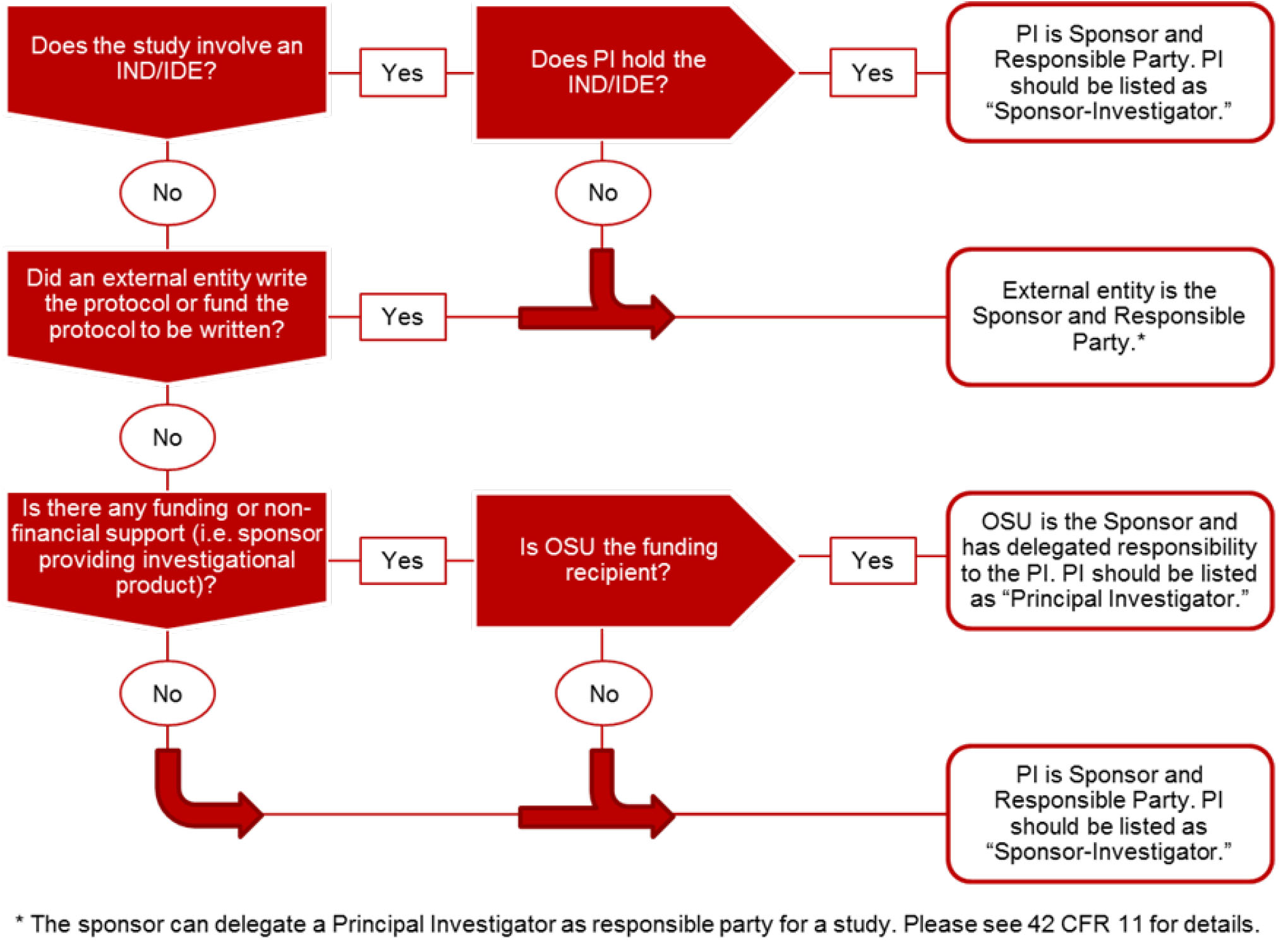
- Responsible Party: The individual with complete access to trial data and rights to publish. The Responsible Party for Investigator Initiated studies in the College of Medicine is the Principal Investigator. The Responsible Party may designate individuals to help complete the ClinicalTrials.gov record, however, the final responsibility of review and approval lies with the Responsible Party. The Responsible Party has the sole authority to release a record.
- Record Owner: This individual can be the Principal Investigator or a designated research team member that is responsible for updating the ClinicalTrials.gov record and ensuring that it is updated in a timely manner. The owner must maintain communication so that the protocol record is released by the PI (Responsible Party) in the required time frame.
- International Committee of Medical Journal Editors (ICMJE): A small group of general medical journal editors and representatives of selected related organizations working together to improve the quality of medical science and its reporting.
- Protocol Registration and Results System (PRS): Website for entering and updating ClinicalTrials.gov records. https://register.clinicaltrials.gov/
- Applicable Clinical Trial (ACT): Registration is required for trials that meet the FDAAA 801 definition of an "Applicable Clinical Trial" and were either initiated after September 27, 2007, or initiated on or before that date and were still ongoing as of December 26, 2007. Trials that were ongoing as of September 27, 2007 and reached their completion date (see Primary Completion Date data element on ClinicalTrials.gov) before December 26, 2007 are excluded. "Applicable Clinical Trials" include the following:
- Procedures
- Determining Study Registration Requirements:
The PI is responsible for determining if the study is an ACT, thus requiring registration and results information on ClinicalTrials.gov. To determine if a study is an applicable clinical trial per 42 CFR 11, please use the checklist available on the ClinicalTrials.gov website
(https://prsinfo.clinicaltrials.gov/ACT_Checklist.pdf).
In addition to federal requirements (42 CFR 11) and the NIH Policy, journals (i.e., ICMJE), funding sources, and insurance companies (i.e., Medicare/Medicaid) may require registration and submission of results information.
If the study was approved by the Cancer IRB, the PI will be instructed to work with appropriately delegated ClinicalTrials.gov Administrator within the Comprehensive Cancer Center to ensure research studies are registered appropriately.
Studies with approved consent language stating the study will be registered to ClinicalTrials.gov must register their study to ClinicalTrials.gov. If it is determined that the study does not require registration per regulations and the PI decides not to register the study for publication or other purposes, then an amendment removing the ClinicalTrials.gov language must be approved by the IRB and consented subjects
must be notified.
Studies with human subjects that are not required per regulations or publication requirements may still be registered to ClinicalTrials.gov per the PI’s discretion. - Determining Responsible Party
Please use the flow diagram and links below, as well as the NIH’s flow sheet and information on the ClinicalTrials.gov website to determine who the Responsible Party is and what option should be selected. For studies initiated and written by an investigator at OSU, the PI of the study should be listed as the Responsible Party, whether listed as “Principal Investigator” or “Sponsor-Investigator.” The Responsible Party has the sole authority to approve and release the record; all records must be reviewed and released by the Principal Investigator. In the event that the PI leaves OSU, please contact the administrators to determine who should become the new Responsible Party.
http://grants.nih.gov/ClinicalTrials_fdaaa/Responsible_Party.htm
http://prsinfo.clinicaltrials.gov/ElaborationsOnDefinitions.pdf
- Obtain an Account
The Ohio State University has separate ClinicalTrials.gov administrators for cancer and non-cancer clinical research studies.- Requests for a ClinicalTrials.gov account can be made by contacting the appropriate organizational ClinicalTrials.gov administrator.
- The requestor must provide full name, preferred institutional e-mail address, IRB number and title of the protocol. If the requestor is not the PI, an e-mail from the PI to the ClinicalTrials.gov administrator is required to create or change access. Please note that the PI of the study will need to have a ClinicalTrials.gov account in order to approve and release the record. You may request that at the same time if the PI does not have an account yet.
- Once an account is created you will be notified by the administrator and will receive an e-mail from the Protocol Registering System (PRS) to modify your password.
- Instructions to create, edit, approve and release a study record will be provided to the PI and record owner.
- Confirm Responsible Party (Refer to section B for the definition of “responsible party”): Use the following guidance flow diagram to determine if who is the responsible party:
https://grants.nih.gov/ClinicalTrials_fdaaa/docs/registration_flow_chart.pdf .
- Create, Update and Maintain Study Records
Instructions for creating, editing, approving, and releasing a study record are available on the main ClinicalTrials.gov website.- Creating and updating submissions: For new records please refer to Attachment A: Creating a New Study Record for ClinicalTrials.gov.
- Maintaining record: please refer to Attachment B: Maintaining Study Records on ClinicalTrials.gov.
- Records release: All records must be reviewed and released by the PI.
- Timeline Requirements
Event Timeline requirements Notes Registration No later than 21 days after the first subject is enrolled
ICMJE requires that registration is complete prior to first subject enrollment.A study is considered registered once the responsible party releases the record to PRS for review. Actively enrolling studies Update/verification every 6 months The record must be verified even if no changes need to be made. Studies closed to enrollment or pending results Update/verification annually The record must be verified even if no changes need to be made. Change in study status Within 30 days of status change
ResultsResults submission No later than 1 year after the primary completion date Delayed submission of results is permitted in certain circumstances. See 42 CFR 11.44 for details. - Protocol Record Management
- The Responsible Party is ultimately responsible for ensuring the studies are registered with ClinicalTrials.gov and updated appropriately at required intervals and released to the public database. Refer to Section D above for timeline requirements for updates.
- The PI and protocol Record Owner will be contacted by the appropriate ClinicalTrials.gov administrator if their protocol record is delinquent and needs to be updated.
- If the protocol record remains delinquent two weeks after the first notice, a second notification will go out to the PI, protocol Record Owner and department chair.
- If the protocol record remains delinquent one month after the initial contact without acceptable activity/progress, the COMOR will arrange for alternative management of the account by another party. The cost of these services will be billed to the department.
- Records that are entered into the ClinicalTrials.gov database, but are not released, will get one notification and if left incomplete at 30 days post notification, the record will be deleted from the database.
- Please note that records cannot be deleted once they have been issued an NCT number, even after the study has been completed. There are limited circumstances when a record can be removed from the public site – please contact the administrator for assistance.
- Transferring a Record:
- If the Record Owner or PI is leaving the Institution they should inform their ClinicalTrials.gov Administrator to ensure the record is appropriately monitored or transferred. The Record Owner or PI can either be reassigned to another Record Owner or PI within the university, or the record can be transferred to a new institution. The ClinicalTrials.gov organizational name for the institution where the PI is transferring should be provided. If this information is unavailable at the time, contact information should be provided to facilitate transfer.
- If the PI (Responsible Party) is moving studies from another institution please contact your designated ClinicalTrials.gov administrator to help facilitate the record transfer.
- Penalties
- Under 42 CFR 11, civil and monetary penalties exist for noncompliance. Monetary penalties can be up to 10,000 US dollars a day.
- Grant funding can be withheld until the required clinical trial information has been submitted.
- Journals can refuse to publish data from records that are noncompliant.
- Noncompliance with OSU policies, 42 CFR 11, and other requirements could result in corrective actions that may include reporting of noncompliance to the IRB.
- Determining Study Registration Requirements:
- Applicable Regulations, Guidances and Policies
Regulation/ Guidance/Policy Title 42CFR11 Final Rule NIH Policy on Clinical Trial Registration What NIH Grantees Need to Know About FDAAA Food and Drug Modernization Act Section 113 Information Program on Clinical Trials for Serious Life-Threatening Diseases Food and Drug Administration Amendments Act (FDAAA) Section 801 Expanded Clinical Trial Registry Data Bank Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects World Health Organization International Clinical Trials Registry Platform
(ICTRP)International Committee of Medical Journal Editors Clinical Trial Registration Food and Drug Administration Amendments Act (FDAAA) Section 801 Elaboration of Definitions of Responsible Party & Applicable Clinical Trial ORRP Guidance Clinical Trials Registration Office of Responsible Research Practices guidance on Clinical Trial Registration
Do you need guidance on whether your human subjects research are subject to FDA regulations? A tool has been developed to assist research teams in determining whether their human subjects research activities are subject to FDA regulations.
Additional Resources
- FDA, FDAAA, Title VIII, Section 801
- FDA, FDAAA, Final Rule
- NIH Final Policy
- NIH “Elaboration of Definitions of Responsible Party and Applicable Clinical Trial”
- FDA 21 CFR § 50.25(c) regulation on informed consent
- FDA informed consent guidance
- Identifying an ACT under FDAAA/NIH flow chart
- Identifying the responsible party under FDAAA/NIH flow chart
- International Committee of Medical Journal Editors “Clinical Trials Registration: A Statement from the International Committee of Medical Journal Editors”, September 2004
- International Committee of Medical Journal Editors “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals”, December 2016
- Centers for Medicare & Medicaid Services “Pub 100-04 Medicare Claims Processing: Transmittal 2955”
CT.gov Points of Contact by College
College of Dentistry
Tonia Gibson
Gibson.49@osu.edu
College of Education and Human Ecology
Kim Lightle
Lightle.16@osu.edu
College of Food, Agricultural, and Environment Science
Melissa Burant
Burant.2@osu.edu
Lori Kaser
Kaser.37@osu.edu
College of Medicine
All Inquiries: COMResearchCompliance@osumc.edu
College of Nursing
Mary Beth Happ
Happ.3@osu.edu
Grace Njoroge
Njoroge.6@osu.edu
College of Optometry
Karla Gengler-Nowak
Gengler-Nowak.1@osumc.edu
College of Pharmacy
Jeanne Green
Green.516@osu.edu
College of Social Work
Lori Blum
Blum.71@osu.edu
Comprehensive Cancer Center
Lisa Brenner
Brenner.79@osu.edu
Office of Research
Sandra Meadows
Meliha Rahmani